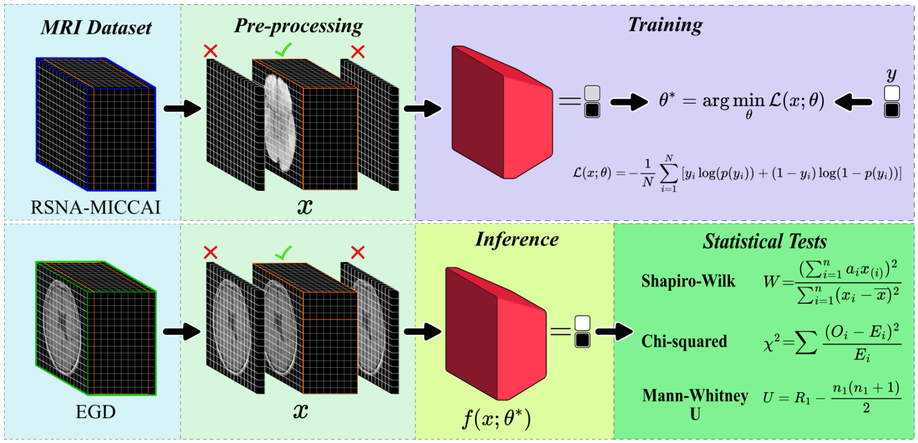
Deep Learning for Glioblastoma Multiforme Detection from MRI: A Statistical Analysis for Demographic Bias
Glioblastoma, IDH-wildtype (GBM), is the most aggressive and complex brain tumour classified by the World Health Organization (WHO), characterised by high mortality rates and diagnostic limitations inherent to invasive conventional procedures. Early detection is essential for improving patient outcomes, underscoring the need for non-invasive diagnostic tools. This study presents a convolutional neural network (CNN) specifically optimised for GBM detection from T1-weighted magnetic resonance imaging (MRI), with systematic evaluations of layer depth, activation functions, and hyperparameters. The model was trained on the RSNA-MICCAI data set and externally validated on the Erasmus Glioma Database (EGD), which includes gliomas of various grades and preserves cranial structures, unlike the skull-stripped RSNA-MICCAI images. This morphological discrepancy demonstrates the generalisation capacity of the model across anatomical and acquisition differences, achieving an F1-score of 0.88. Furthermore, statistical tests, such as Shapiro–Wilk, Mann–Whitney U, and Chi-square, confirmed the absence of demographic bias in model predictions, based on p-values, confidence intervals, and statistical power analyses supporting its demographic fairness. The proposed model achieved an area under the curve–receiver operating characteristic (AUC-ROC) of 0.63 on the RSNA-MICCAI test set, surpassing all prior results submitted to the BraTS 2021 challenge, and establishing a reliable and generalisable approach for non-invasive GBM detection.
A Review of Artificial Intelligence-Based Systems for Non-Invasive Glioblastoma Diagnosis
Glioblastoma multiforme (GBM) is an aggressive brain tumor with a poor prognosis. Traditional diagnosis relies on invasive biopsies, which pose surgical risks. Advances in artificial intelligence (AI) and machine learning (ML) have improved non-invasive GBM diagnosis using magnetic resonance imaging (MRI), offering potential advantages in accuracy and efficiency. Objective: This review aims to identify the methodologies and technologies employed in AI-based GBM diagnostics. It further evaluates the performance of AI models using standard metrics, highlighting both their strengths and limitations. Methodology: In accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, a systematic review was conducted across major academic databases. A total of 104 articles were identified in the initial search, and 15 studies were selected for final analysis after applying inclusion and exclusion criteria. Outcomes: The included studies indicated that the signal T1-weighted imaging (T1WI) is the most frequently used MRI modality in AI-based GBM diagnostics. Multimodal approaches integrating T1WI with diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) have demonstrated improved classification performance. Additionally, AI models have shown potential in surpassing conventional diagnostic methods, enabling automated tumor classification and enhancing prognostic predictions.
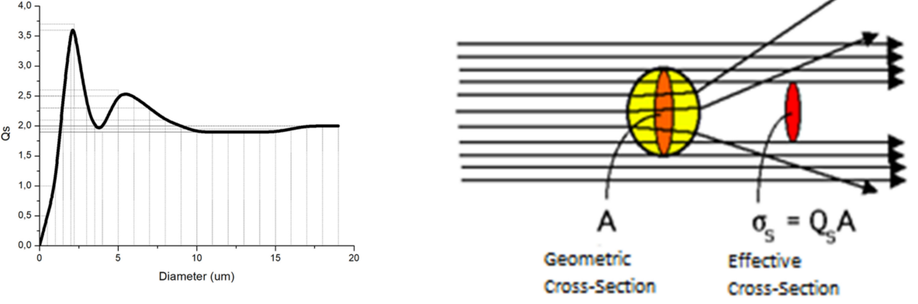
The use of silica microparticles to improve the efficiency of optical hyperthermia (OH)
Although optical hyperthermia could be a promising anticancer therapy, the need for high concentrations of light-absorbing metal nanoparticles and high-intensity lasers, or large exposure times, could discourage its use due to the toxicity that they could imply. In this article, we explore a possible role of silica microparticles that have high biocompatibility and that scatter light, when used in combination with conventional nanoparticles, to reduce those high concentrations of particles and/or those intense laser beams, in order to improve the biocompatibility of the overall procedure. Our underlying hypothesis is that the scattering of light caused by the microparticles would increase the optical density of the irradiated volume due to the production of multiple reflections of the incident light: the nanoparticles present in the same volume would absorb more energy from the laser than without the presence of silica particles, resulting either in higher heat production or in the need for less laser power or absorbing particles for the same required temperature rise. Testing this new optical hyperthermia procedure, based on the use of a mixture of silica and metallic particles, we have measured cell mortality in vitro experiments with murine glioma (CT-2A) and mouse osteoblastic (MC3T3-E1) cell lines. We have used gold nanorods (GNRs) that absorb light with a wavelength of 808 nm, which are conventional in optical hyperthermia, and silica microparticles spheres (hereinafter referred to as SMSs) with a diameter size to scatter the light of this wavelength. The obtained results confirm our initial hypothesis, because a high mortality rate is achieved with reduced concentrations of GNR. We found a difference in mortality between CT2A cancer cells and cells considered non-cancer MC3T3, maintaining the same conditions, which gives indications that this technique possibly improves the efficiency in the cell survival. This might be related with differences in the proliferation rate. Since the experiments were carried out in the 2D dimensions of the Petri dishes, due to sedimentation of the silica particles at the bottom, whilst light scattering is a 3D phenomenon, a large amount of the energy provided by the laser escapes outside the medium. Therefore, better results might be expected when applying this methodology in tissues, which are 3D structures, where the multiple reflections of light we believe will produce higher optical density in comparison to the conventional case of no using scattering particles. Accordingly, further studies deserve to be carried out in this line of work in order to improve the optical hyperthermia technique.
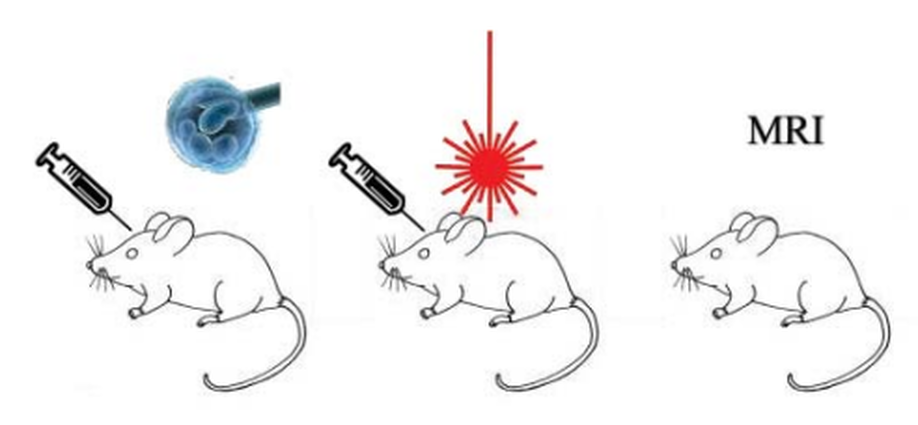
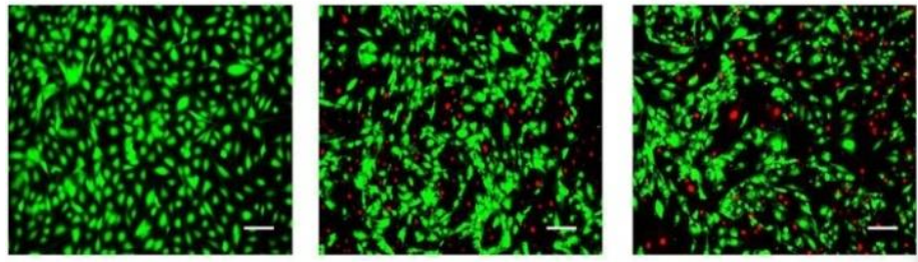
Slowdown intracranial glioma progression by optical hyperthermia therapy: Study on a CT-2A mouse astrocytoma model
Metallic nanorods are promising agents for a wide range of biomedical applications. We report an optical hyperthermia method capable of inducing slowdown tumor progression of an experimental in vivo CT-2A glioblastoma tumor. The tumor model used in this research is based on the transplantation of mouse astrocytoma CT-2A cells in the striatum of mice by intracranial stereotaxic surgery. Two weeks after cell implant, the resulting tumor is treated by irradiating intratumoral injected gold nanorods, biofunctionalized with CD133 antibody (B-GNRs), using a continuous wave laser. Nanoparticles convert the absorbed light into localized heat (reaching up to 44 °C) due to the effect of surface plasmon resonance. A significant slowdown in CT-2A tumor progression is evident, by histology and magnetic resonance imaging, at one (p = 0.03) and two weeks (p = 0.008) after irradiation treatment. A notable deceleration in tumor size (15%–75%) as compared to the control untreated groups, it is observed. Thus, laser irradiation of B-GNRs is found to be effective for the treatment of CT-2A tumor progression. Similarities between the pre-clinical CT-2A tumor model and the human astrocytoma disease, in terms of anatomy, metastatic behavior and histopathology, suggest that hyperthermic treatment by laser irradiation of B-GNRs administered into high-grade human astrocytoma might constitute a promising alternative treatment to limit the progression of this deadly disease.
Influence of medium viscosity and intracellular environment on the magnetization of superparamagnetic nanoparticles in silk fibroin solutions and 3T3 mouse fibroblast cell cultures
Magnetic hyperthermia mediated by superparamagnetic particles is mainly based in sinusoidal waveforms as excitation signals. Temperature changes are conventionally explained by rotation of the particles in the surrounding medium. This is a hypothesis quite questionable since habitual experimental setups only produce changes in the magnetic module, not in the field lines trajectories. Theoretical results were tested by changing the waveform of the exciting signal in order to compare non-sinusoidal signals against sinusoidal signals. Experiments were done at different frequencies: 200 KHz, 400 KHz, 600 KHz, 800 KHz and 1 MHz. Superparamagnetic Iron Oxide samples (SPION), made of magnetite (Fe3O4) and suspended in water (100 mg/ml), were used. Magnetic field strength varies from 0.1±0.015 KA/m to 0.6±0.015 KA/m. In this study was observed that the power loss depends on the applied frequency: for 1 to 2.5 RMS current the responses for each signal are part of the higher section of the exponential function, and for 3.5 to 8 RMS current the response is clearly the decrement exponential function’s tale (under 1× 103 LER/gr).
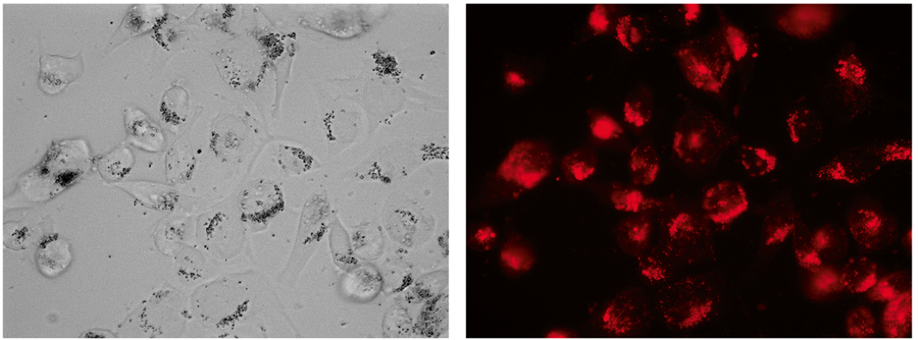
Induction of cell death by magnetic particles in response to a gradient magnetic field inside a uniform magnetic field
A new instrument based on a magnetic force produced by an alternating magnetic field gradient, which is obtained through Maxwell coils, inside a constant field magnet has been designed and used to produce cell death. We have determined the interaction of microparticles and cells under different conditions such as incubation time with microparticles, particle size, magnetic field exposition time, and different current waveforms at different frequencies to produce a magnetic field gradient. We determined that the highest rate of cell death occurs at a frequency of 1 Hz with a square waveform and 1 h of irradiation. This method could be of great interest to remove cancer cells due mainly to the alterations in stiffness observed in the membranes of the tumor cells. Cancer cells can be eliminated in response to the forces caused by the movement of magnetic nanoparticles of the appropriate size under the application of a specific magnetic field.
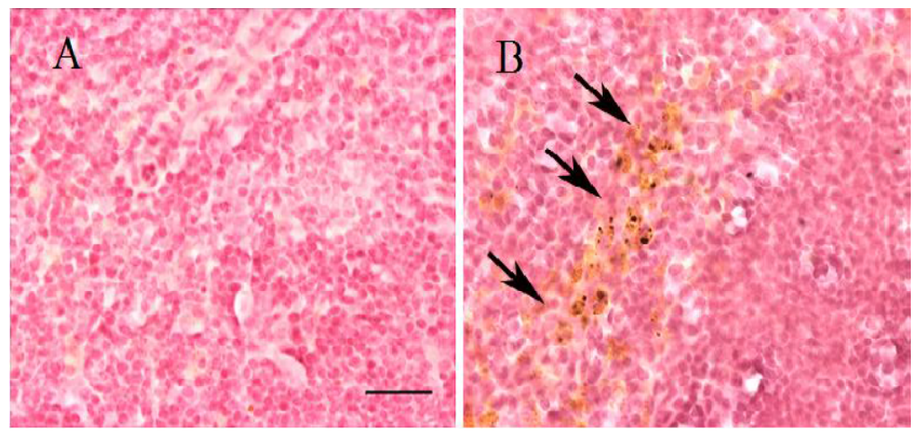
Contribución al estudio de las propiedades de las nanopartículas magnéticas en aplicaciones biomédicas relacionadas con la detección en tejidos ex-vivo y cultivos celulares
Since its original conception, nanotechnology has emerged as a leading area of research offering significant benefits to society through applications and technological developments that have revolutionized various areas of science. In the health sector, the use of nanotechnology, also known as nanomedicine, has benefited the health system in areas of diagnosis and therapy though the introduction of more efficient, localized, and personalized techniques. The use of Magnetic Nanoparticles (MNPs) for biomedical purposes has generated a great interest due to their unique physical properties, which can lead to new therapeutic methods. However, there is a need for understanding the magnetic nanoparticles properties and their interactions with living organisms to develop safer and more efficient therapies. Moreover, the lack of studies focused on the analysis of nanoparticles distribution and final destination in the body after a therapy, in addition to the toxicological problems that it can generate, has slowed down the commercial acceptance of many therapies that utilize nanomaterials. This situation is due both to an underestimation of the study of nanotoxicology and to the difficulty of implementing effective monitoring and detection techniques. Therefore, this thesis seeks to give a direct answer to these issues still completely unresolved. For this reason, an exhaustive measurement protocol was designed in order to carry out an appropriate characterization of MNPs distributed in biological tissues from experimental mouse models, which a dose of MNPs was previously administered. Additionally, the characterization of MNPs suspended in viscous fluids (deionized water and fibroin solutions) and internalization into cultured cells were performed. Through this study, also intends to demonstrate and to assess the feasibility of the measurement methods as an useful tool to perform the study of the magnetic behavior of the MNPs suspended into viscous fluids and their internalization into cell cultures and ex-vivo tissues. In order to carry out the magnetic characterization of the nanoparticles, the Laboratory of Bioinstrumentation and Nanomedicine (LBN) of the Centre for Biomedical Technology (CTB) of the Technical University of Madrid (UPM), has a platform of functional characterization of MNPs. This platform corresponds to Unit 15 of the system NANBIOSIS-ICTS driven by the Centre for Biomedical Research Network Bioengineering, Biomaterials and Nanomedicine (CIBER- BBN) to promote the biotechnology research. The Unity of functional characterization of MNPs is composed by two devices: an Alternating Gradient Magnetometer MicroMagTM 2900 (AGM System) and a Fast Field Cycling Relaxometry (FFCR). The saturation magnetization of the magnetic materials is measured by an AGM. Their high sensibility and precision make these especially attractive to characterize MNPs. The relaxation times and dispersion profiles of the MNPs are measured with the FFCR. It is important to mention that an adaptation of the AGM’s probe was necessary through the manufacturing of a sample holder with specific characteristics to carry out the study of the magnetic behavior of nanoparticles suspend in viscous fluids and internalized into cell cultures. The results obtained showed the validity of the procedures since it was possible to efficiently observe differences in the magnetic behavior of the nanoparticles according to the time and type of tissue. Overall, it was noticed that after the injection, the nanoparticles travel through the bloodstream during the first hours and over time these are eliminated via urine or deposited in some organs. One month after administrating the injection of the MNPs, an accumulation of nanoparticles was detected in the spleen and liver, thus indicating a response of the reticuloendothelial system (RES). However, in the brain was not detected any accumulation of MNPs during the time the study last (1 month), indicating that MNPs did not cross the blood-brain barrier (BBB). On the other hand, a decrease in the saturation magnetization of the MNPs was observed depending on the viscosity of the medium and the concentration of the magnetic material. This change is caused by the dipole-dipole interactions, the formation of clusters or chains, and the hindrance of magnetic moments to align with the magnetic field by increasing the viscosity of the medium and the concentration. Furthermore, it was possible to detect a decrease in the saturation magnetization of the nanoparticles when they were internalized into the lysosomes. The variation caused by the encapsulation of the MNPs in the lysosomes causes a decrease in the degrees of freedom of the magnetic moments of the MNPs and the formation of aggregates. On the other hand, an increase in the relaxation induced by the increased viscosity of the fluid was observed because the surface of the MNPs is protected against free water by the fibroin matrix. This leads us to conclude that fibroin matrix could be a good coating agent for MNPs. In general, the viability of Unit 15 was verified as an efficient and versatile tool to carry out studies on the biodistribution and monitoring of MNPs through the study of ex-vivo tissues. Likewise, this tool allows research on the physical properties of MNPs suspended in viscous fluids and internalized into cell cultures.
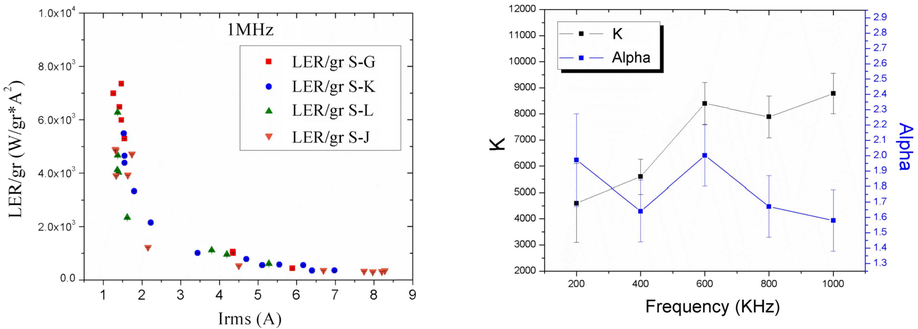
Study of the dependency of the specific power absorption rate on several characteristics of the excitation magnetic signal when irradiating a SPION-containing ferrofluid
Magnetic hyperthermia mediated by superparamagnetic particles is mainly based in sinusoidal waveforms as excitation signals. Temperature changes are conventionally explained by rotation of the particles in the surrounding medium. This is a hypothesis quite questionable since habitual experimental setups only produce changes in the magnetic module, not in the field lines trajectories. Theoretical results were tested by changing the waveform of the exciting signal in order to compare non-sinusoidal signals against sinusoidal signals. Experiments were done at different frequencies: 200 KHz, 400 KHz, 600 KHz, 800 KHz and 1 MHz. Superparamagnetic Iron Oxide samples (SPION), made of magnetite (Fe3O4) and suspended in water (100 mg/ml), were used. Magnetic field strength varies from 0.1±0.015 KA/m to 0.6±0.015 KA/m. In this study was observed that the power loss depends on the applied frequency: for 1 to 2.5 RMS current the responses for each signal are part of the higher section of the exponential function, and for 3.5 to 8 RMS current the response is clearly the decrement exponential function’s tale (under 1× 103 LER/gr).
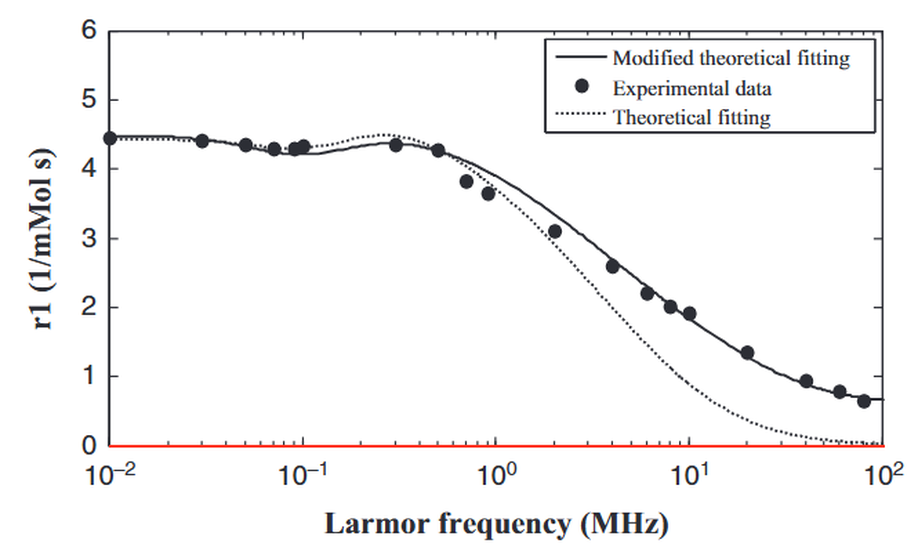
Assessment of a heuristic model for characterization of magnetic nanoparticles as contrast agent in MRI
In magnetic resonance imaging (MRI), the use of magnetic nanoparticles (MNPs) as contrast agent (CA) greatly enhances the possibility to identify several diseases hardly diagnosed by other means. The efficacy of a new CA is described by the longitudinal and transverse relaxivity. Nuclear Magnetic Relaxation Dispersion (NMRD) profiles represent the evolution of relaxivities with magnetic field. Many efforts have been taken to develop theoretical models to depict water proton relaxation in presence of magnetic compounds. The use of theoretical models in junction with NMRD profiles has become a powerful tool to characterize MNPs as CA. In this work, a heuristical theoretical model was implemented, verified and assessed with different magnetic materials. It has been demonstrated that the model works well when using iron cores but fails with other magnetic compounds. A weighting factor associated with Langevin function was introduced to the model. This extra calibration enables the model to be used with other magnetic compounds to characterize new CAs in MRI.
A Comparison of Magnetometry and Relaxometry Measures of Magnetic Nanoparticles Deposited in Biological Samples
The Alternating Gradient Field Magnetometer (AGFM) is an instrument whose high sensitivity (10-8 emu) allows the detection of small amounts of magnetic nanoparticles (MNPs) with high accuracy. Over the last few years, different magnetic techniques have been used for in vitro measurements of magnetic nanostructures inside biological tissues. However, in vivo studies about their distribution within the body are very scarce because their dispersion, after being delivered, reduces their magnetic signal and hinders detection. In this paper we compare the longitudinal relaxation time (T1) and magnetization measurements in mice’s biological tissues for the tracking of MNPs after of an injection of iron oxide nanoparticles. Furthermore, we have correlated the AGFM data with Fast Field Cycling NMR Relaxometry (FFCNMR Relaxometry) measurements with histological analysis. The results have demonstrated that these techniques are useful for detecting minute amounts of MNPs in excised organs after in-vivo comparable to other more conventional techniques for the measurement of MNPs biodistribution and clearance. Details about the preparation of the in vivo samples, measurement protocol and statistical data processing are given.
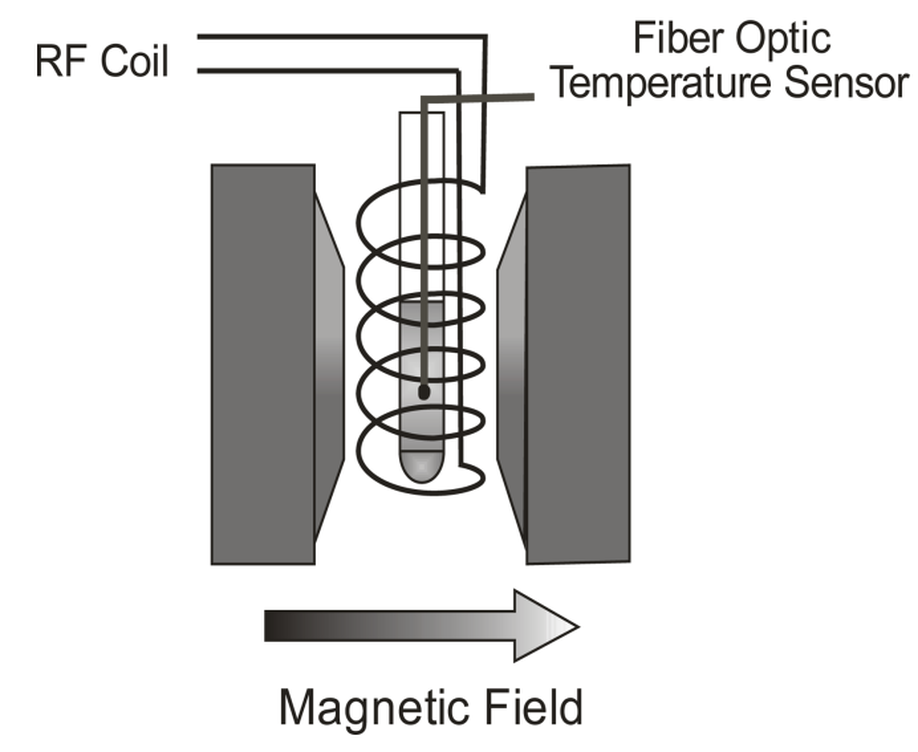
Power absorption measurements during NMR experiments
The heating produced by the absorption of radiofrequency (RF) has been considered a secondary undesirable effect during MRI procedures. In this work, we have measured the power absorbed by distilled water, glycerol and egg-albumin during NMR and non-NMR experiments. The samples are dielectric and examples of different biological materials. The samples were irradiated using the same RF pulse sequence, whilst the magnetic field strength was the variable to be changed in the experiments. The measurements show a smooth increase of the thermal power as the magnetic field grows due to the magnetoresistive effect in the copper antenna, a coil around the probe, which is directly heating the sample. However, in the cases when the magnetic field was the adequate for the NMR to take place, some anomalies in the expected thermal powers were observed: the thermal power was higher in the cases of water and glycerol, and lower in the case of albumin. An ANOVA test demonstrated that the observed differences between the measured power and the expected power are significant.